By Austin Weber, Senior Editor // webera@bnpmedia.com
New Process Improves Lifespan of Lithium-Ion Batteries
AV/EV tech trends
A new manufacturing method developed in South Korea can strengthen lithium-ion battery anodes, making them more resilient against volume changes. Illustration courtesy Gwangju Institute of Science and Technology
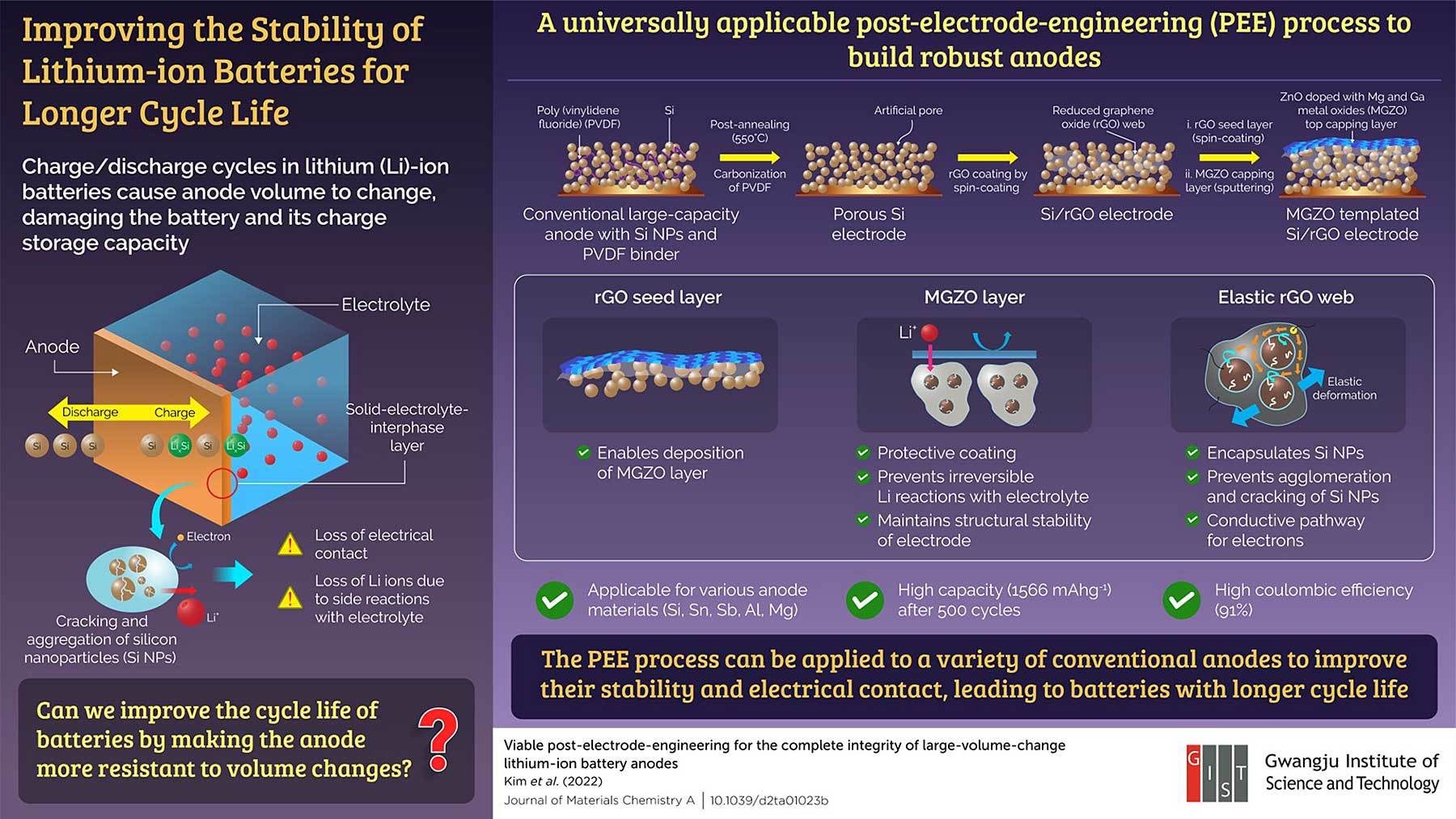
Charging and discharging a lithium-ion battery multiple times causes significant change to the volume of its anode, which reduces battery capacity and life cycle. To address the issue, engineers at the Gwangju Institute of Science and Technology (GIST) have developed a method to strengthen the anode, making it more resilient against volume changes. The anode can be modified irrespective of its material or how it is manufactured, and could lead to long-lasting electric vehicle batteries.
The short life cycle of traditional lithium-ion batteries results from an irreversible volume change in the anode during cycling, which causes degradation of electrical contacts and structural collapse. During charging, lithium ions move from the cathode and combine with the nanoparticles in the anode.
During discharging, the lithium ions move back to the cathode. Over time, the nanoparticles in the anode crack and cluster together at the electrode-electrolyte interface. This causes an electrical disconnection, reducing the amount of charge the anode can store or transport.
GIST engineers developed a way to strengthen the anode and make it more resilient against volume changes by encapsulating the nanoparticles in an elastic web-like structure. They used a conventional anode containing silicon nanoparticles held together by a polymer (polyvinylidene fluoride) binder. To accommodate the web-like structure, the engineers removed the binder by heating the anode using an annealing process.
“The gap between the nanoparticles was then filled in with reduced graphene oxide (rGO) solution, which dried up to form a web that held the silicon nanoparticles together and prevented them from cracking,” says Hyeong-Jin Kim, Ph.D., a chemistry professor at GIST who is head of the Energy Storage Material and Device Lab. “Additionally, the web provided a conductive pathway for the electrons, allowing the nanoparticles to bind with lithium.”
Kim and his colleagues used spin coating to coat the anode surface with rGO. Next, they deposited a protective layer consisting of zinc oxide with magnesium and gallium metal oxides (MGZO) added to it. This layer provided structural stability to the anode.
The modified anode was able to retain most of its charge even after several charge-discharge cycles. “The structure retained a high storage capacity of 1,566 mA h g-1 after 500 cycles and showed 91percent coulombic efficiency, which relates to the battery life,” explains Kim. “This could pave the way for electric vehicles that enable us to drive long distances on one-time charging.”
Although the engineers used a silicon anode, their method can be used with other anode materials, such as aluminum, antimony, magnesium and tin. In addition, the anodes can be modified regardless of how they are manufactured, making it a universally applicable way to improve battery life.
Extra ‘Eye’ Movements May Be Key to Safer Autonomous Vehicles

Artificial neural networks that learn to recognize objects faster and more accurately may enable autonomous vehicles to navigate better. Illustration courtesy AutoSens
Engineers at the Riken Center for Brain Science in Wako, Japan, have developed a way to create artificial neural networks that learn to recognize objects fast and accurately. It can be applied to machine vision technology, making it easier for autonomous vehicles to learn how to recognize important features on the road.
“Despite making constant head and eye movements throughout the day, objects in the world do not blur or become unrecognizable, even though the physical information hitting our retinas changes constantly,” says Andrea Benucci, Ph.D., head of the Laboratory for Neural Circuits and Behavior. “What likely makes this perceptual stability possible are neural copies of the movement commands. These copies are sent throughout the brain each time we move and are thought to allow the brain to account for our own movements and keep our perception stable.”
In addition to stable perception, evidence suggests that eye movements, and their motor copies, might also help people recognize objects in the world, but how this happens remains a mystery.
Benucci and his colleagues developed a convolutional neural network (CNN) that solves this challenge. The CNN was designed to optimize the classification of objects in a visual scene while the eyes are moving.
First, the network was trained to classify 60,000 black and white images into 10 categories. Although it performed well on these images, when tested with shifted images that mimicked naturally altered visual input that would occur when eyes move, performance dropped drastically to chance level.
However, classification improved significantly after training the network with shifted images, as long as the direction and size of the eye movements that resulted in the shift were also included. In particular, adding the eye movements and their motor copies to the network model allowed the system to better cope with visual noise in the images.
“This advancement will help avoid dangerous mistakes in machine vision,” claims Benucci. “With more efficient and robust [technology], it is less likely that pixel alterations will cause self-driving cars to label a stop sign as a light pole or military drones to misclassify a hospital building as an enemy target.
“The benefits of mimicking eye movements…while informing the vision network in charge of processing the associated images about the self-generated movements would make machine vision more robust and akin to what is experienced in human vision,” explains Benucci.
Longer Lasting Sodium-Ion Batteries Are on the Horizon
A new sodium-ion battery is stable and can operate at high voltages. Photo courtesy Pacific Northwest National Laboratory
Cheap and abundant, sodium is a promising candidate for new EV battery technology. But, limited performance of sodium-ion batteries in the past has hindered large-scale applications.
Engineers at Pacific Northwest National Laboratory (PNNL) have a solution. They recently developed a battery with greatly extended longevity due to a change in the ingredients that make up the liquid core of the device.
The engineers changed the liquid solution and the type of salt flowing through it to create a new electrolyte recipe. In laboratory tests, the design proved durable, holding 90 percent of its cell capacity after 300 cycles at 4.2 volts, which is higher than most sodium-ion batteries previously reported.
The current electrolyte recipe for sodium-ion batteries results in the protective film on the negative end (the anode) dissolving over time. This film is critical, because it allows sodium ions to pass through while preserving battery life.
The PNNL technology works by stabilizing this protective film. The new electrolyte also generates an ultra-thin protective layer on the positive pole (the cathode) that contributes to additional stability of the entire unit.
The sodium-ion battery uses a natural fire-extinguishing solution that is also impervious to temperature changes and can operate at high voltages. One key to this feature is the ultra-thin protective layer that forms on the anode. It remains stable once formed, providing long cycle life.
“We also measured the production of gas vapor at the cathode,” says Phung Le, Ph.D., a scientist at PNNL who specializes in electrochemical materials and systems. “We found very minimal gas production. This provides new insights to develop stable electrolyte for sodium-ion batteries that may operate at elevated temperatures.”
For now, the sodium-ion technology still lags behind lithium in energy density. However, it has its own advantages, such as imperviousness to temperature changes, stability and long cycle life. Le and her colleagues are also experimenting with other designs in an effort to reduce—and eventually eliminate—the need to include cobalt, which is toxic and expensive if not recovered or recycled.
