SCROLL
DOWN
Michael R. Taylor, J.D., will receive Food Safety Magazine's Distinguished Service Award for 2023. The award will be presented at the 2023 Food Safety Summit, taking place May 8–11 in Rosemont, Illinois.
Mr. Taylor has held leadership roles in public health and food safety in government, academia, and the private sector, with a major focus on modernizing the U.S. food safety system to prevent foodborne illness. He led the U.S. Food and Drug Administration's (FDA's) role in the legislative enactment of the Food Safety Modernization Act (FSMA) of 2011, and currently co-chairs the board of Stop Foodborne Illness, a consumer organization supporting and representing the victims of foodborne illness and their families.
From 2010–2016, he served as the Deputy Commissioner for Foods and Veterinary Medicine at FDA. In addition to his work on FSMA, he oversaw FDA's other food-related activities, including its nutrition, labeling, food additive, dietary supplement, and animal drug programs. Previously, Mr. Taylor was appointed Administrator of the U.S. Department of Agriculture's Food Safety and Inspection Service (USDA's FSIS) in 1994, where he overhauled the FSIS program to establish legal accountability for industry to prevent contamination of meat and poultry with pathogenic bacteria, particularly E. coli O157:H7. He also led FDA's new Office of Policy from 1991–1994, where he worked on the implementation of the Nutrition Labeling and Education Act of 1990.
Prior to joining FDA in 2009, he spent nearly a decade in academia conducting food safety, food security, and public health policy research, most recently at George Washington University's School of Public Health. Mr. Taylor is a graduate of Davidson College and the University of Virginia School of Law.
Past recipients of the Food Safety Magazine Distinguished Service Award include Reginald Bennett, M.Sc., Dane Bernard, M.Sc., Larry Beuchat, Ph.D., Robert L. Buchanan, Ph.D., Frank Busta, Ph.D., John N. Butts, Ph.D., Darin Detwiler, Ph.D., Keith Ito, Allen Katsuyama, Connie Kirby, M.Sc., John W. Larkin, Ph.D., Huub Lelieveld, Barbara Masters, D.V.M., Ann Marie McNamara, Ph.D., Theodora Morille-Hinds, M.Sc., William Sperber, Ph.D., Joe Stout, R.S., Steve Taylor, Ph.D., David Theno, Ph.D., Bruce Tompkin, Ph.D., and Don L. Zink, Ph.D.

Taylor to Receive Food Safety Magazine Distinguished Service Award
FDA Announces Vision for Restructuring Human Foods Program; Stakeholder Groups Levy Criticism
Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images

At the end of January 2023, the U.S. Food and Drug Administration (FDA) announced a reorganization plan for the agency's Human Foods Program, as well as for the Office of Regulatory Affairs (ORA), to better support FDA as a whole. The plan includes creating a unified Human Foods Program under a single leader who reports directly to the Commissioner of FDA, removing redundancies and enabling the agency to oversee human food in a more effective and efficient way.
The agency's decision comes after reviewing the findings and recommendations from an external evaluation conducted by the Reagan-Udall Foundation, as well as a separate internal review of the agency's infant formula supply chain response completed last year. Under the new plan, the functions of the Center for Food Safety and Applied Nutrition (CFSAN), the Office of Food Policy and Response (OFPR), and certain functions of ORA will be unified into a newly envisioned organization called the Human Foods Program. FDA will conduct a competitive national search for a Deputy Commissioner for Human Foods who will oversee the program. Other key elements of the proposed reorganization include:
- The creation of a Center for Excellence in Nutrition that prioritizes FDA's ongoing efforts to help American consumers make more informed food choices. FDA proposes to establish an Office of Critical Foods, as directed by the 2023 Consolidated Appropriations Act, within this center.
- The establishment of an Office of Integrated Food Safety System Partnerships that will focus on elevating, coordinating, and integrating FDA's food safety and response activities with state and local regulatory partners to more effectively meet the vision of an Integrated Food Safety System, as envisioned in FSMA.
- To help support the agency's scientifically grounded decision-making activities, a Human Foods Advisory Committee will be established and consist of external experts to advise FDA on challenging and emerging issues in food safety, nutrition, and innovative food technologies.
- There will be an emphasis on strengthening FDA's enterprise information technology and analytical capabilities to fulfill the promise described in the New Era of Smarter Food Safety and support the improvement in workflow that will accompany these changes.
- As part of the proposed new vision, ORA's operating structure will be transformed into an enterprise-wide organization that supports the Human Foods Program and all other FDA regulatory by focusing on its critical activities—inspections, laboratory testing, import, and investigative operations. The change is anticipated to improve risk prioritization and the public health impact of FDA's field activities, modernize FDA's field activities, and create operational efficiencies. Boots-on-the-ground inspectors will remain within ORA, but the priorities and budget for human foods-related activities will be the direct responsibility of the new Deputy Commissioner of Human Foods.
- While the FDA's Center for Veterinary Medicine (CVM) will continue to operate as a standalone center, the relevant food safety activities will be closely coordinated between the CVM Center Director and the Deputy Commissioner for Human Foods.
- FDA has formed an Implementation and Change Management Group that will develop a detailed plan to ensure the successful execution of the new vision.
On February 28, FDA announced a national search for a new Deputy Commissioner for Human Foods and provided an update on the recently proposed restructuring. Steps FDA has taken to achieve the new vision for the agency include:
- Assessing specific functions of ORA, CFSAN, and OFPR to be unified into a new Office of Integrated Food Safety Systems Partnerships that will engage with state, local, tribal, and territorial food safety regulatory partners, taking into consideration how best to enhance connectivity with international food safety partnership programs.
- Analyzing inspection and compliance functions that sit within both ORA and program offices across the agency to determine opportunities to streamline operations and clarify decision-making authority at each step of the inspection process, as well as to integrate new automation and information technology support.
- Determining how best to empower the Deputy Commissioner for Human Foods and leaders of other programs, along with the Associate Commissioner for Regulatory Affairs, to oversee program and field resource allocation, including publicly mapping the budget to functional activities to provide clarity on resource allocation.
- Ensuring coordination across FDA and state-operated food laboratory operations by evaluating the foods laboratory programs, including the relationships, roles, and responsibilities among CFSAN, CVM, ORA, and state-operated laboratories.
- Improving FDA's ability to conduct risk prioritization by performing an evaluation of how the Human Foods Program accomplishes risk management, particularly risk prioritization, given the multitude of demands and the scarce resources, and how this can be used to guide dynamic work planning and resource allocation.
- Planning for greater enterprise transformation of certain ORA IT functions, which will be coordinated with the FDA's Office of Digital Transformation (ODT), building on an existing project to create an enterprise-wide platform for managing inspections and compliance activities.
- Evaluating training programs, including for FDA investigators, to see how they can best serve the needs of FDA, regulatory partners, and industry.
Following the announcement, organizations representing consumers, industry, and state and local regulators expressed their concerns. The coalition of stakeholder organizations—including Consumer Reports, Consumer Brands Association (CBA), the Association of Food and Drug Officials (AFDO), Stop Foodborne Illness, the International Fresh Produce Association (IFPA), the Environmental Working Group, and the American Frozen Food Institute (AFFI)—has been asking FDA to strengthen leadership at the agency by appointing a fully empowered Deputy Commissioner for foods, with direct line authority over all key elements of the Foods Program, as recommended in the Reagan-Udall Foundation's report. The coalition of stakeholder organizations believe that FDA's plan rejects the Reagan-Udall Foundation's recommendation that the agency appoint an empowered, single leader of the Human Foods program. Coalition representatives shared their concerns in a media briefing attended by Food Safety Magazine on February 28.
Overall, the stakeholder representatives expressed that they have not felt included in effective dialogue about the restructuring at FDA thus far. When asked what the next steps are for the stakeholder groups, Roberta Wagner, Vice President of Regulatory and Technical Affairs at the Consumer Brands Association, stated that they would have "no choice but to go to congress," where the coalition believes there is bipartisan support to enact meaningful changes to FDA's Human Foods program.
FDA's search for a new Deputy Commissioner for Human Foods is underway, and is open to both external and internal candidates under the agency's expanded Title 21 hiring authority for a foods-related position. FDA is seeking to finalize its proposal by autumn 2023, including the newly designed structure, an established budget, and a detailed mapping and crosswalk of staff from the current to new organization.
The Codex Alimentarius Committee on Food Hygiene (CCFH) has released an amended proposed draft guidance on the management of foodborne illness outbreaks associated with microbiological hazards. The draft guidance is intended to advise food safety authorities on the preparedness and management of foodborne illness outbreaks of microbiological origin, including on communication with international networks like the International Food Safety Authorities Network (INFOSAN) and notification to the World Health Authority (WHO) under the International Health Regulations (IHR). The guidance addresses incident preparedness, detection, and response to limit the extent of outbreaks as much as possible. It also recommends the appropriate use of new analytical technologies, such as genetic typing methods, during an outbreak investigation.
Also described in the guidance are the roles of food safety authorities at the local, national, and international level, and the collaboration among authorities in official network structures. Guidelines are included on collaboration and communication with food business operators and other stakeholders before and during foodborne illness outbreaks, as well as during post-outbreak activities and outbreak management reviews. Maintenance of structures and training methods to strengthen network response are also addressed.

Codex Publishes Guidance on Managing Microbiological Foodborne Illness Outbreaks
FDA Releases New Food Fraud Webpage
The U.S. Food and Drug Administration (FDA) has released a new website on economically motivated adulteration (EMA), including food fraud. The purpose of the website is to keep businesses and consumers informed on the latest food fraud developments.
The website includes links on how to report food fraud; examples of food adulteration; how food fraud is detected and monitored; enforcement and legal consequences, such as recalls, seizures, and import refusals; guidance documents to assist manufacturers and importers; and a list of import alerts.
EMA occurs when "someone intentionally leaves out, takes out, or substitutes a valuable ingredient or part of a food," according to FDA. EMA also occurs when a substance is added to a food to make it appear better or of greater value.
Food fraud is a common type of EMA that FDA investigates, but EMA also occurs with other products, including animal food and cosmetics. Some types of EMA are also misbranding violations. Estimating how frequently food fraud occurs or its exact economic impact can be challenging because food fraud is designed to avoid detection. Outside estimates by experts have found that food fraud affects 1 percent of the global food industry at a cost of approximately $10–$15 billion per year, although more recent expert estimates peg the cost as high as $40 billion per year.
Food fraud can also lead to major health issues and even death. Some examples include lead poisoning from adulterated spices and allergic reactions to a hidden or substituted ingredient that contains a small amount of just one food allergen.
Click here to visit FDA’s new EMA website.

Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images
The U.S. Department of Agriculture's Food Safety and Inspection Service (USDA's FSIS) has released its Strategic Plan for Fiscal Years (FY) 2023–2026. The FSIS Strategic Plan is the foundation document for both the long-range and day-to-day operations of the agency.
Goal 1: Prevent Foodborne Illness and Protect Public Health
The first goal aims to prevent adulteration and misbranding, as well as to limit foodborne illnesses that occur because of FSIS-regulated products. Objectives identified by FSIS under Goal 1 include:
- Strengthen compliance with food safety statutes and regulations
- Achieve pathogen reduction
- Ensure labeling is truthful and not misleading
- Strengthen food safety practices throughout the supply chain
- Enhance collaborative response to foodborne illness outbreaks and other public health incidents
- Raise consumer awareness of food safety.
Goal 2: Transform Inspection Strategies, Policies, and Scientific Approaches to Improve Public Health
The desired outcomes of Goal 2 are to improve food safety through the adoption of innovative approaches and technologies, and to optimize data use at every level of agency decision-making. Objectives identified by FSIS under Goal 2 include:
- Advance and adopt innovative regulatory policies and inspection verification procedures
- Foster the adoption of advanced scientific techniques
- Improve the integrity, accessibility, and utility of data
- Strengthen data analyses and evaluations
- Optimize the design of sampling programs for decision-making.
Goal 3: Achieve Operational Excellence
The desired outcomes of Goal 3 are to sustain and advance an adaptable, high-performing, and engaged workforce, as well as to optimize service delivery. Objectives identified by FSIS under Goal 3 include:
- Expand recruitment and increase retention for mission-critical positions
- Enhance employee training and professional development
- Ensure equal opportunity, civil rights, diversity, equity, inclusion, and accessibility in the work environment
- Enhance effectiveness and efficiency of key business procedures
- Improve customer service
- Transform business infrastructure and information technology.
FSIS will implement the Strategic Plan by using its performance management framework, which includes monitoring and reporting processes supported by the agency's enterprise governance process. FSIS will regularly track and monitor progress, ensure FSIS meets intended targets, and make timely and necessary adjustments to key activities or approaches.

USDA-FSIS Strategic Plan for 2023–2026 Focuses on Innovation, Reducing Foodborne Illness
FDA has announced new recommended action levels for lead in certain processed baby foods. The proposed action levels support the Closer to Zero initiative to continually reduce babies' and young children's exposure to toxic heavy metals from food.
The FDA draft guidance, titled, Action Levels for Lead in Food Intended for Babies and Young Children, covers processed foods—such as products packaged in jars, pouches, tubs, and boxes—intended for babies and children less than two years of age. Lead may be present in the aforementioned foods because the agricultural commodities the products are derived from—fruits, vegetables, grains, and animals—take up contaminants in the environment, as well as nutrients.
The draft guidance sets the following action levels:
- 10 parts per billion (ppb) for fruits, vegetables (excluding single-ingredient root vegetables), mixtures (including grain and meat-based mixtures), yogurts, custards and puddings, and single-ingredient meats
- 20 ppb for single-ingredient root vegetables
- 20 ppb for dry cereals.
FDA estimates that the new action levels could reduce babies' and young children's exposure to lead from the covered foods by 24–27 percent. Action levels are not intended to set the lowest levels for industry to achieve, but are meant to cause manufacturers to implement agricultural and processing measures to lower lead levels in food products.
Although not binding, FDA can reference proposed action levels when considering whether to bring enforcement action in a particular case. For all foods, with or without action levels, the agency will take action when it finds that the level of lead in a food is unsafe, which may include working with the manufacturer to resolve the issue and removing product from the U.S. market. FDA will monitor industry's progress in reducing the levels of lead in the foods identified in the draft guidance, while ensuring that manufacturers are putting in place any needed preventive controls to reduce or eliminate the presence of lead in products.

FDA Sets Action Levels for Lead in Baby Foods as Part of Closer to Zero Initiative
USDA's FSIS has expanded its generic label approval regulations to include certain categories of meat, poultry, and egg products, beginning March 19, 2023. The final rule, published on January 18, 2023, also responds to comments received on the September 2020 proposal.
The final rule expands generic approval to the labels of products that are only intended for foreign commerce, even if such labels deviate from U.S. labeling requirements; labels of products that receive voluntary FSIS inspection; labels with "geographic landmarks"; labels with "negative" claims that identify the absence of certain ingredients or types of ingredients; and labels with "organic" claims in the ingredients statement. FSIS will continue to require prior approval of labels that display "organic" claims outside the ingredients statement, including those certifying a total product as organic. FSIS will no longer evaluate generically approved labels that establishments voluntarily submit for FSIS review. FSIS has also announced the availability of the revised FSIS Guideline for Label Approval for the types of labels that must be submitted to FSIS for approval.

USDA-FSIS Updates Generic Label Approval Regulations
We lost a great friend, colleague, and expert food safety leader this year, Peter Good. He was an amazing leader, teacher, and developer of training concepts and materials. Peter made complex food safety requirements and methods approachable and easy to understand. He did not only teach food safety to restaurant owners/operators and managers so they could pass certification exams; he also produced an outcome of understanding and enthusiasm so they could apply what they learned to ensure food safety in their business. Everyone who listened passed his exams. Peter would travel around the U.S. to educate business leaders on food safety, and we would only hear great things from all his students, many of whom were business leaders. Peter could teach just about anything in a way that the knowledge could be applied. He established a successful business based on this gift, and we are sure that his training induced a significant reduction in food safety risk in many restaurants across the nation.
Hal King, Ph.D.
Active Food Safety
Steven A. Lyon, Ph.D.
Chick-fil-A Inc.

Industry Marks the Passing of Peter Good

The International Association for Food Protection (IAFP) has announced that Lisa Hovey, former IAFP Assistant Director, has assumed the Executive Director role. Previous Executive Director David Tharp has retired after 30 years with the association.
Harpreet S. Kochhar, Ph.D., has been appointed President of the Canadian Food Inspection Agency (CFIA).
Frank Yiannas resigned as Deputy Commissioner of Food Policy and Response at FDA on February 24, 2023. Donald Prater, Ph.D., is serving as Acting Director of the Office of Food Policy and Response as of the time of publication.
International Fresh Produce Association's (IFPA) Chief Food Safety and Regulatory Officer, Jennifer McEntire, Ph.D., will step away from her role with the association on May 5, 2023.
Mike Durkin, President and Chief Executive Officer of the Leprino Foods Company, was elected Chair of the board of directors of the checkoff-founded Innovation Center for U.S. Dairy. He replaces Schreiber Foods Chair Mike Haddad, who served as the Innovation Center's chair for five years.
Impossible Foods has hired top consumer goods industry leader Sherene Jagla as its first Chief Demand Officer.
PPG named Polina Ware as its new Packaging Coatings Global Technical Director in January 2023.
Duda Farm Fresh Foods has promoted Mark Bassetti, former Chief Operating Officer and Senior Vice President of Duda Farm Fresh Foods, as the subsidiary's new President. Bassetti's promotion follows the previous appointment of Samuel Duda as the new CEO of DUDA, the parent company of Duda Farm Fresh Foods.
IFT Board President, Chris Downs, Ph.D., has assumed a new role as Director of the Food and Beverage Accelerator (FaBA) Trailblazer program funded by the Australian Government Department of Education and hosted by The University of Queensland.
The National Association of State Departments of Agriculture (NASDA) Foundation has hired Chris Jones as Senior Director of the NASDA Foundation. Also, NASDA has announced Oklahoma Department of Agriculture Secretary Blayne Arthur as Vice President of the organization.
Brandon Headlee has been promoted to Senior Director of Food Safety at Conagra Brands Inc.
Church Brothers Farms has appointed Victor Soto as Food Safety Manager and Maria Chavarin as Food Safety Specialist.
Katerina Mastovska, Ph.D., has been named Deputy Executive Director and Chief Science Officer at AOAC International.
Marlen International Inc., a member of the Duravant family of operating companies, has appointed Einar Einarsson to President.
AAK, an ingredient supplier specializing in value-adding specialty fats and oils, has hired Rini Roy as Product Manager.

THARP

MCENTIRE

DURKIN

JAGLA

WARE

BASSETTI
Raslysation Could Replace Pasteurization, Uses UV for Microbial Inactivation of Liquids
Biotech group Novozymes is making the change from classic filtration of its industrial enzyme liquids to a raslysation system from Danish company Lyras, which inactivates microbial contaminants in liquid foods using ultraviolet (UV) technology. Raslysation can be used as a substitute for the pasteurization of foods such as brine, whey, juice, iced tea, and many other liquids. Novozymes' new raslysation system will be able to treat 45,000 liters of industrial enzyme liquid per hour. Raslysation is a non-thermal treatment technology based on UV that inactivates microorganisms but is gentle on enzymes, proteins, amino acids, and vitamins. Raslysation provides higher quality, increased safety, improved production flow, less cleaning and waste, and better energy efficiency.

Energis Solutions™ has released results from a recent validated study confirming its pathogen reduction technology, Guardian™, which is highly effective in reducing common pathogens found in the wheat tempering process. The study shows that Guardian performs at a 225 percent higher kill rate than acid-based solutions, without leaving chemical residues or altering the functionality of wheat and flour. As part of the study, Hard Red Winter wheat was inoculated with Salmonella and Escherichia coli, and was then treated using Energis Solutions' Guardian treatment technology. Results showed an average 5.4 log reduction of E. coli and an average 3.93 log reduction of Salmonella when analyzed against the non-treated control samples.
Technology Achieves Pathogen Reduction Milestone in Wheat Temper

Food packaging manufacturer Sabert Corporation has announced that it intends to eliminate all intentionally added per- and polyfluoroalkyl substances (PFAS) from its full product portfolio by the end of 2023. The company's molded fiber and paper portfolio currently features over 85 moisture- and oil-resistant products manufactured without intentionally added PFAS, including more than 40 that are certified by the Biodegradable Products Institute. More than 75 percent of Sabert's fiber products already meet PFAS-free standards. Furthering Sabert's ability to eliminate PFAS from all products is the opening of its newest manufacturing facility in Greenville, Texas, which is developing exclusively PFAS-free packaging products for the North American market and beyond.
Sabert Commits to Eliminating PFAS from All Products by End-2023


ONLINE & OF NOTE
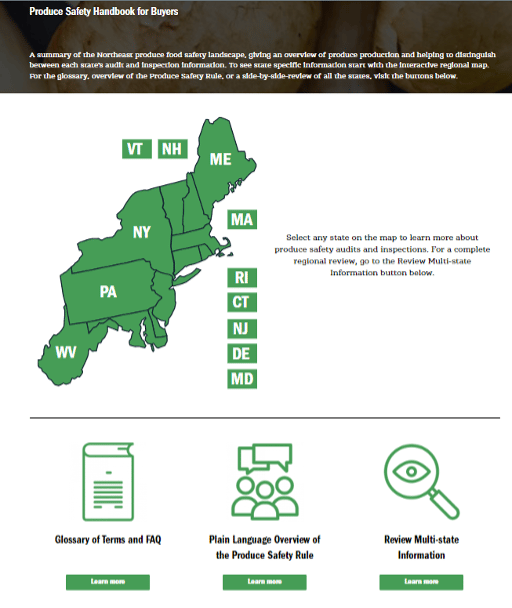
The Northeast Center to Advance Food Safety (NECAFS) recently announced its new online Produce Safety Handbook for Buyers. The handbook is formatted as an interactive website that clarifies the complex landscape of food safety regulations and standards across 12 different states in the U.S. Northeast. The Produce Safety Handbook helps produce buyers navigate each state's produce safety audit and inspection information. The site provides an interactive regional map, farm production statistics by state, a glossary of terms with common audit and inspection language, state inspection and audit information depicted with simple charts and tables, and regional content in a side-by-side display.
States included in the handbook are Maine, Massachusetts, New Hampshire, Vermont, New York, Rhode Island, Connecticut, Delaware, New Jersey, Pennsylvania, West Virginia, and Maryland. Previously, NECAFS published an online food safety toolkit for food processors, tailored to small and very small processors that are required to comply with FDA's Preventive Controls for Human Food Rule under FSMA.