SANITATION
By Manjeet Sharan, M.V.Sc., Pankaj Dhaka, Ph.D., and J.S. Bedi, Ph.D.
Biofilm: A Contemporary Challenge to Food Safety
Biofilms, which can be resistant to disinfectants and sanitizers, remain a significant public health-related issue in the food industry

Image credit: thelinke/E+ via Getty Images
SCROLL DOWN
Biofilm is a colonial structure formed by microorganisms in order to survive and adapt in adverse environmental conditions. In nature, biofilms alternate in two modes of growth: planktonic (free-floating state) and sessile (when microorganism adhere to the surface). Diverse species of microorganisms are involved in biofilm formation, such as bacteria (e.g., Pseudomonas, Staphylococcus, E. coli, etc.), fungi (e.g., Candida, Aspergillus) and protozoa (e.g., amoebae, heliozoa).
Biofilms produce extracellular polymeric substance (EPS) known as "slime," which comprise polysaccharides, proteins, phospholipids, teichoic acids, and nucleic acids. In most biofilms, the matrix comprises more than 90 percent of the dry mass, while the microorganism accounts for less than 10 percent. The EPS provides protection to the microbial cells against environmental stressors by forming a mushroom-shaped protective barrier surrounding the microbial colonies, thereby favoring the growth of biofilm formation.
The formation of biofilm is a dynamic process and generally consists of five developmental stages,1 as presented in Figure 1 and outlined below:
- Reversible attachment of planktonic cell to surface
- Irreversible attachment to form monolayer on surface
- Early development of biofilm architecture by forming multilayer structure and microcolonies
- Maturation step is characterized by formation of mushroom-shaped structure formed by multilayer microcolonies
- Dispersion in which some planktonic cells detach from the biofilm to start a new cycle.
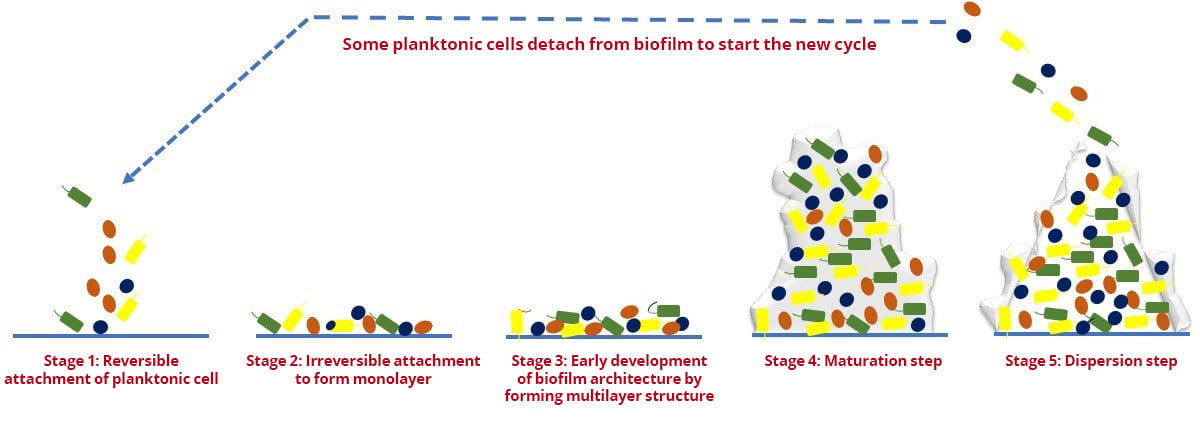
FIGURE 1. Different Developmental Stages of Biofilms in Microorganisms
Microbial cells within biofilm remain in close proximity to one another so that they can interact through the chemicals that enable them to coordinate and respond to any environmental, ecological and host-related adverse conditions. To communicate, microorganisms within biofilm use quorum-sensing (QS) systems. QS is a mechanism opted by bacteria to monitor the cell density inside biofilm and regulate the collective gene behaviors.
In summary, biofilm development gives microbial cells numerous advantages, including physical resistance against drying and/or desiccation; mechanical protection against various types of processing units; and chemical fortification toward different chemicals, disinfectants, and antimicrobials used in the food industry.
Impacts of Biofilm on Food Industry
Biofilm remains a significant public health-related issue in the food industry. The group behavior of pathogens results in resistant behaviors, including for commonly used disinfectants and antibiotics. Through the food supply chain, these pathogens can easily enter into the human and animal populations, making it imperative to understand the biofilm formation dynamics of these pathogens and how to prevent and control their formation. Most foods of animal and plant origin are rich in nutrients, which provide a suitable medium for multiplication of many pathogens. Some of the common pathogens associated with biofilms that have been isolated from various foods are highlighted in Table 1.2

TABLE 1. Microorganisms Associated with Biofilm Potential Isolated from Food Items
Large-scale food production systems provide conducive environments for biofilm formation on food processing lines and contact surfaces, owing to the long production cycles and complex processing facilities. Food contamination can occur at any stage of food processing, from food production to consumption, and can also occur due to cross-contamination from the processing environment. The main sites for biofilm development depend on the type of food operation and processing equipment (e.g., dairy processing plant, ice cream plant, meat processing plant, caviar processing unit, shrimp and fish factory, etc.). The growth surfaces in equipment used in such facilities may include water or other liquid pipelines, floor contact surfaces, pasteurizer plates, equipment and utensils, reverse osmosis membranes, dispensing tubing tables, conveyor belts, feeding units, employee gloves, animal carcasses, storage silos, packaging materials, etc.
The biofilms formed by certain bacteria in food processing units can lead to transmission of disease, food contamination, and/or spoilage of food products (e.g., release of proteolytic and lipolytic enzymes associated with biofilms of Pseudomonas spp. and Bacillus spp., which may create unpleasant smells and bitter tastes), as well as corrosion of metal surfaces in pipes and tanks, which ultimately result in loss of food production surfaces. In addition to being the source of contamination, biofilms also depreciate the efficiency of production in the food processing chain by damaging equipment. These disturbances not only constitute a major financial loss to the food industry, but may also affect the health of consumers by compromising food safety.
Biofilm and Antimicrobial Resistance
The emergence of antimicrobial resistance in microorganisms remains one of the greatest public health challenges, and the formation of biofilm has been postulated as a mechanism of these microorganisms to resist antimicrobial agents.3 When planktonic cells transform into a sessile form, the biofilm resistance develops as a complex phenomenon due to relatively increased gene expression among the pathogens in communities. Formation of biofilms and extracellular polymeric substances (EPS) matrices can enhance resistance toward antimicrobials and disinfectants/sterilizing agents by avoiding drug diffusion and activity at bactericidal concentrations, making these microorganisms immune to eradication and control.
It has been estimated that the microorganisms in biofilms can withstand 10 to 1,000 times higher antimicrobial doses than those required to inactivate similar microbes in their planktonic state.4 Infections or illnesses related to biofilms are often difficult to treat and result in serious public health hazards. The antibiotic resistance of biofilm depends on various factors such as physical, biological, multispecies interactions, and gene-associated factors. This multifactorial nature of biofilm toward drug tolerance imposes greater risks toward the use of conventional antibiotics in food animals and public health. To summarize, bacterial biofilm plays a key role in antimicrobial resistance development. Moreover, the choice of antimicrobials must be significant and effective, as some of them may act as agonists and actually enhance the biofilm activity, while others may disrupt it.
“Novel or advanced control strategies must be developed to discourage microorganisms from building biofilm-associated resistance toward commonly used disinfectants and sanitizers in food processing environments.”


Strategies to Control and Prevent Biofilm Formation
Biofilms associated with the food industry and processing chain can cause considerable damage to human health, and effective techniques must be used to reduce, prevent, and eliminate biofilm microorganisms found on food and food contact surfaces. Cleaning and disinfection procedures are the most widely used methods. The food processing industry traditionally relies on cost-effective physical methods (e.g., heat shock treatment, hot water steam, ozone, or mechanical removal techniques) and chemical methods (e.g., sodium hydroxide or sodium hypochlorite solutions) for the prevention or elimination of biofilm development on food contact surfaces or in the environment.
However, the complete removal of biofilms cannot be accomplished using only conventional methods. Novel or advanced control strategies must be developed to discourage microorganisms from building biofilm-associated resistance toward commonly used disinfectants and sanitizers in food processing environments. Such novel strategies should incorporate the use of the following types of technology:
- Bacteriophages
- Bacteriocins
- Quorum-sensing inhibitors such as paraoxonases (which destroy quorum-sensing signal molecules)
- Essential oils such as cinnamaldehyde, carvacrol, citral, or thymol (which reduce bacterial biofilms from various surfaces)
- Biosurfactants such as lichenysin (can be added to industrial detergents)
- Enzymatic disruptions (use of detergents containing proteases, glycosidases, or DNase)
- Steel surface modification (by coating with silver, copper, or zinc nanoparticles, or by using the novel antibiofilm polymers with lysozyme or bacteriocins)
- High hydrostatic pressure
- Non-thermal plasma to reduce and eliminate pathogens from food environments.5
A "hurdle approach" combines two or more techniques for biofilm control and could prove more effective for tackling biofilm growth in a food processing environment. The combination of different techniques might target the biofilm microbes using different mechanisms of action, which might result in the efficient removal of biofilm growth. It is important to remember that good hygiene and manufacturing practices remain key to reducing the chances of contamination and biofilm development on food contact surfaces.
Conclusion and Way Forward
Biofilms are omnipresent in nature, and the food industry is susceptible to the risks presented by biofilm formation. Biofilms not only impact food manufacturing operations, but also pose a public health risk through the contamination of food and food products. A better understanding of the characteristics of biofilm formation by microorganisms in the food processing chain is essential for the prevention and control of contamination in food processing environments.
Therefore, the development of standard cleaning and disinfection programs, hygienic design for equipment and utensils to prevent and control biofilm, and the subsequent monitoring of their effectiveness should be given priority. The identification of biofilm-prone areas in a food processing environment and the systematic monitoring of microbial load in these areas can help prevent the development of biofilm. Emphasis should be placed on understanding the mechanism of biofilm formation on different food matrices and developing novel, cost-effective technologies to reduce and eliminate biofilm development in the food industry.
References
- Srey, S., I. K. Jahid, and S. D. Ha. "Biofilm formation in food industries: A food safety concern." Food Control 31, no. 2 (2013).
- Shi, X. and X. Zhu. "Biofilm formation and food safety in food industries." Trends in Food Science & Technology 20, no. 9 (2009).
- Rabin, N., Y. Zheng, C. Opoku-Temeng, Y. Du, E. Bonsu, and H. O. Sintim, "Agents that inhibit bacterial biofilm formation." Future Medicinal Chemistry 7, no. 5 (2015).
- Abebe, G. M., "The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination." International Journal of Microbiology (2020).
- Galie, S., C. García-Gutiérrez, E. M. Miguélez, C. J. Villar, and F. Lombó. "Biofilms in the food industry: Health aspects and control methods." Frontiers in Microbiology 9 (2018).
Manjeet Sharan, M.V.Sc., Pankaj Dhaka, Ph.D., and J. S. Bedi, Ph.D. work at the Centre for One Health at the College of Veterinary Science at Guru Angad Dev Veterinary and Animal Sciences University in Ludhiana, India.

