HEAT & CORROSION RESISTANT MATERIALS/COMPOSITES
galitskaya/iStock / Getty Images Plus via Getty Images
Sigma-Phase Metallurgy
Marc Glasser – Rolled Alloys; Temperance, Mich.
Heat treaters are continuously looking for new ways and new alloys that will improve performance at lower cost.
This leads to the stainless steel realm of alloys, including the historical favorite 310 stainless, as well as relative newcomer 253MA®. These alloys have some exceptional qualities, particularly the 253MA alloy, which has usable creep strength to and above 2000°F (1093°C) and oxidation resistance up to 2000°F. 800H is another alloy that exhibits excellent creep strength. In fact, 800H has one of the highest allowable temperature limits, according to the ASME code, of any alloy. However, there is one compelling disadvantage of using such alloys that heat treaters must be cognizant of. That factor is sigma-phase formation over time.
Some investigations have concluded that sigma-phase embrittlement was involved in premature life in nickel alloys when, in fact, sigma phase does not precipitate in nickel alloys. In such cases, the actual microstructure showed grain-boundary oxidation. This article will provide a better understanding of sigma-phase metallography and the effect of sigma phase on stainless steels.
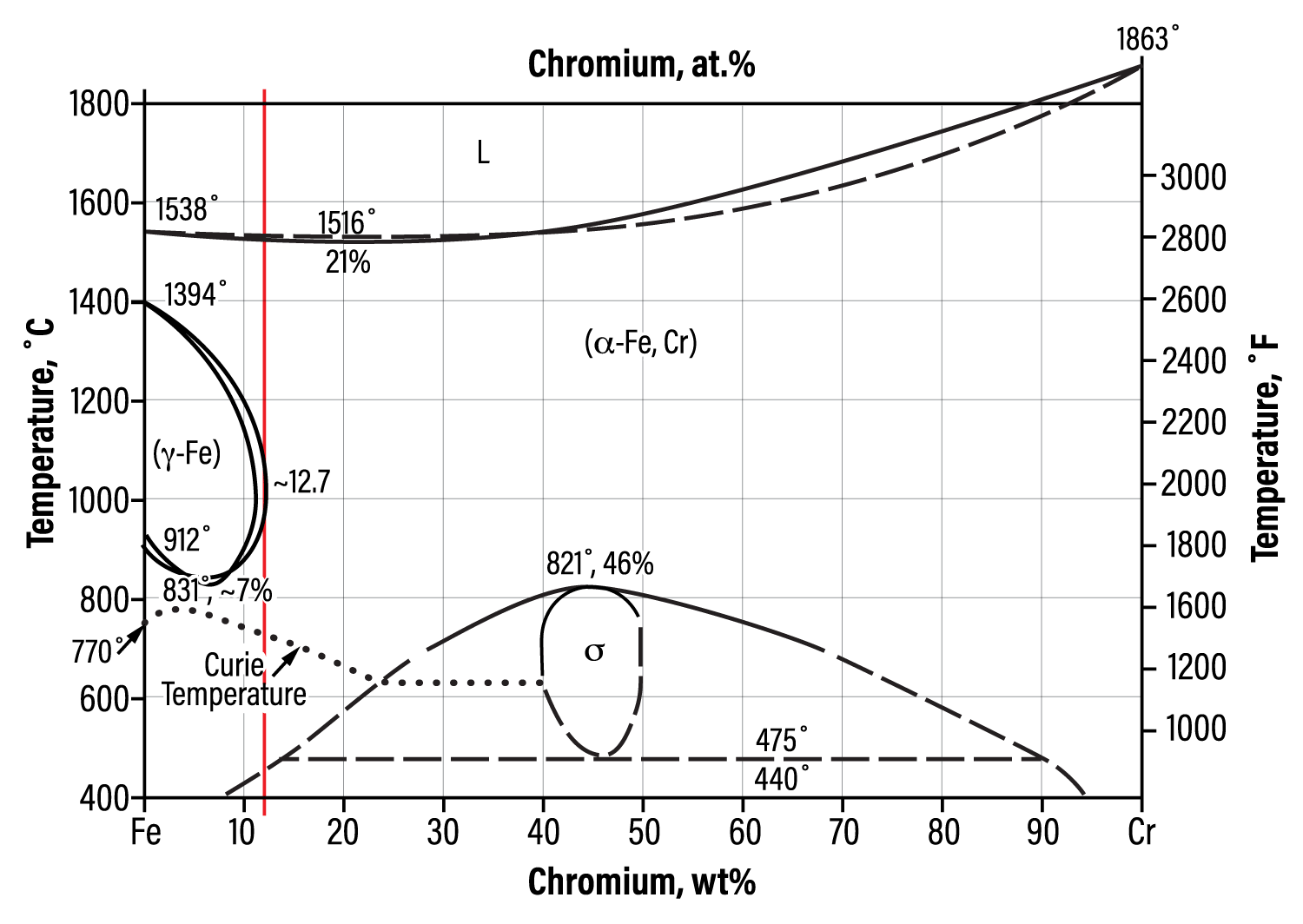
Fig. 1. Iron-chromium phase diagram
Definition of Sigma Phase
Sigma phase is an intermetallic compound consisting of chromium and iron, which is hard, brittle and non-magnetic. At room temperature, sigma phase embrittles material to the point that a sudden, hard impact can shatter a piece of metal containing sigma phase like a piece of glass. Pure sigma forms between 42% and 50% chromium and is one of the equilibrium phases in the iron-chromium phase diagram[1,2] (Fig. 1).
The peak temperature for sigma-phase formation at 46% Cr is 1510°F (821°C). A literature review has different sources citing differing sigma-phase formation temperature ranges. Some of the variation is attributed to each alloy having its own unique sigma formation range. One expert suggests that sigma phase forms in the 1100-1600°F (595-875°C) temperature range.
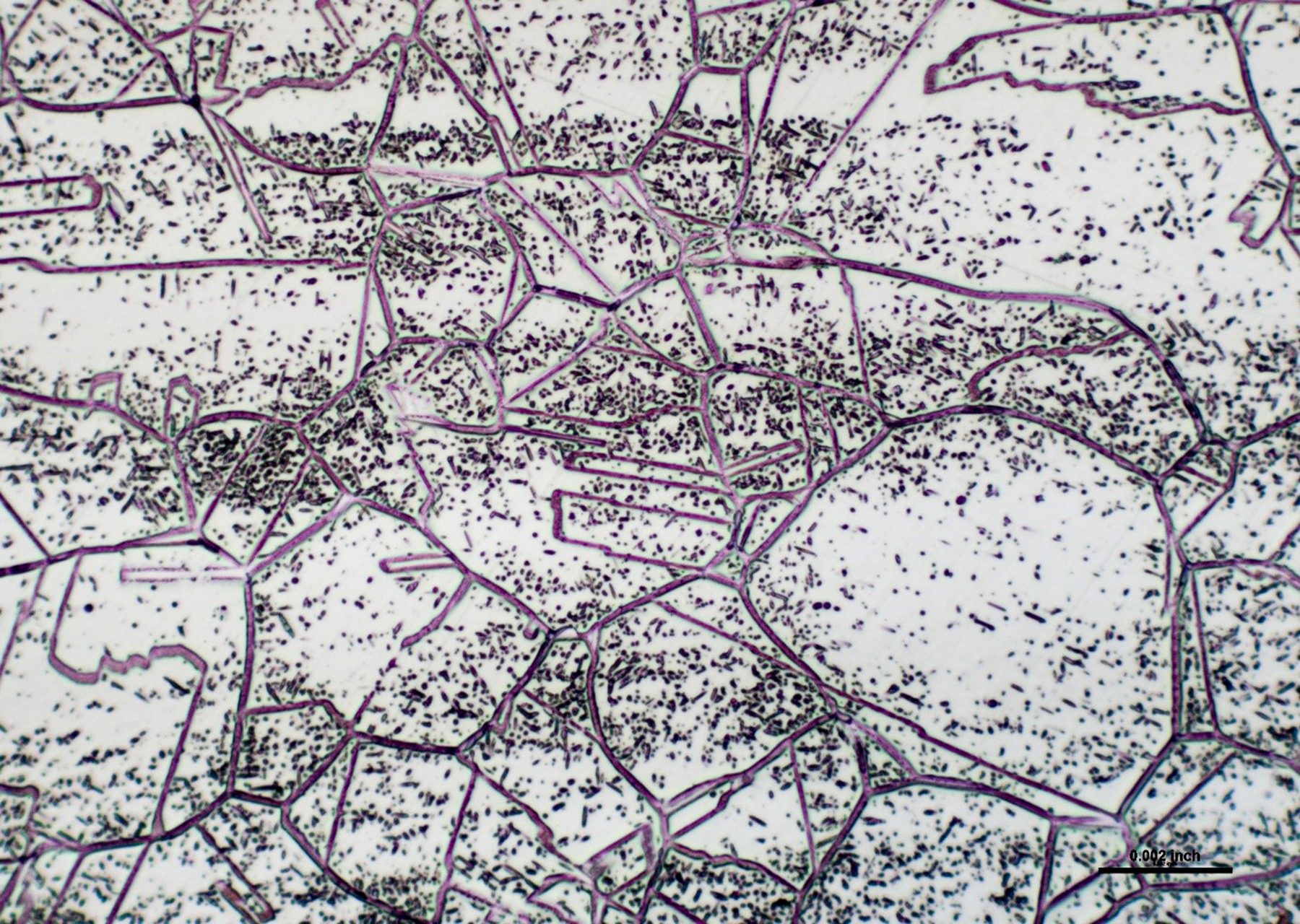
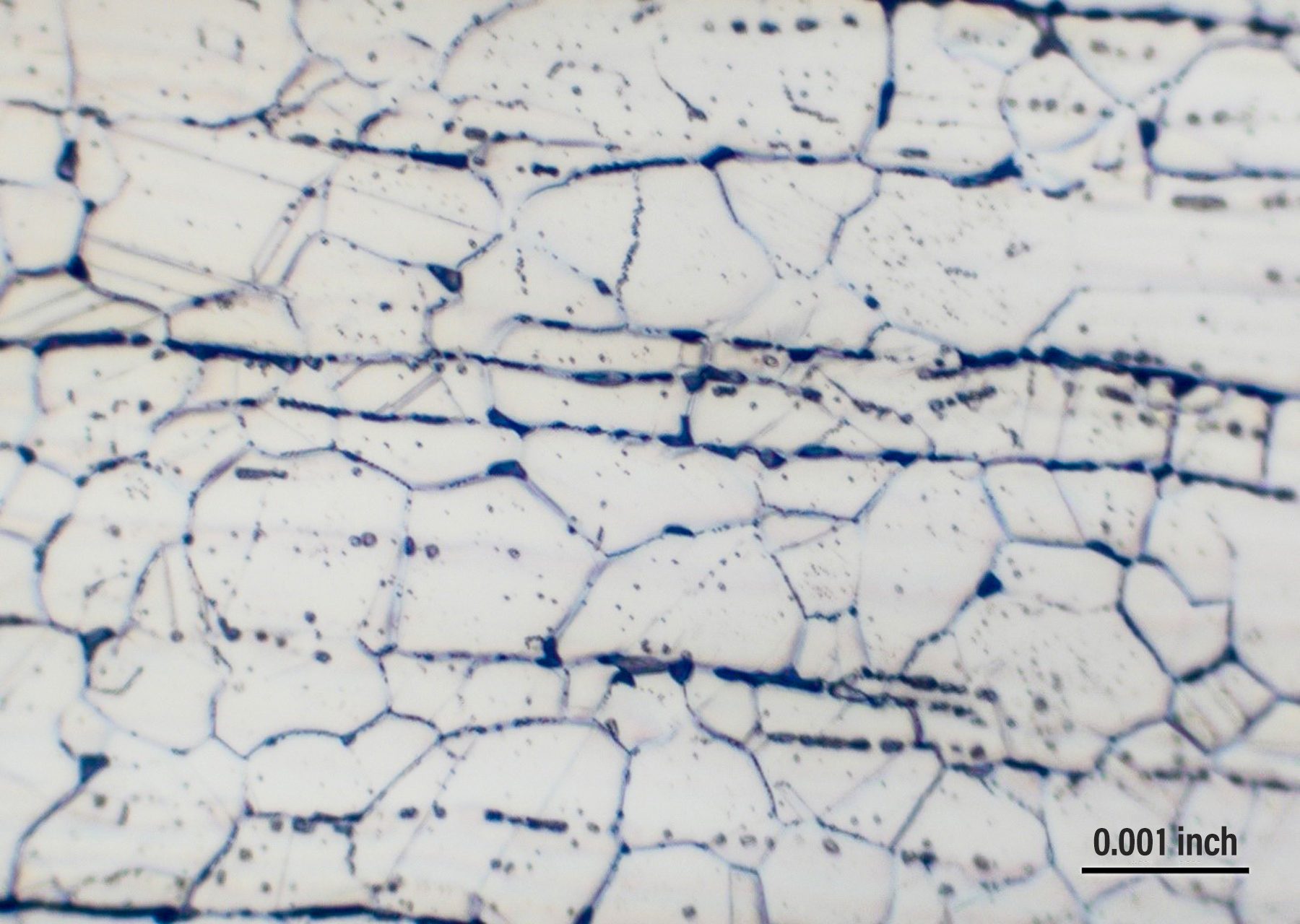
Fig. 2. 310 stainless steel – sigma phase precipitates throughout the structure (3 months at 1450°F; 200x)
Fig. 3. 304L stainless steel – sigma phase precipitates throughout the structure (3 months at 1450°F; 500x)
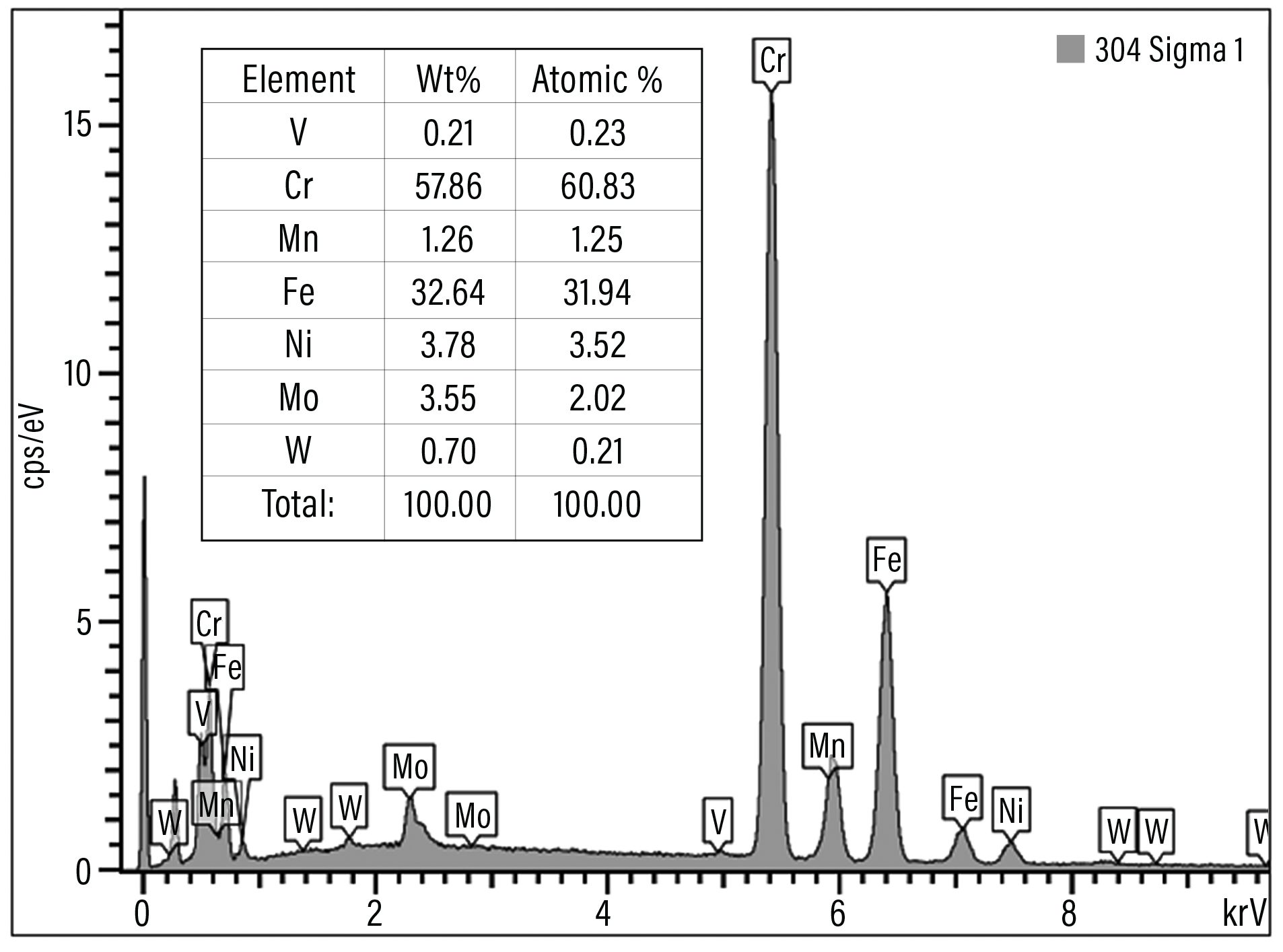
Fig. 4. EDX spectrum showing the composition of sigma phase.
Metallurgy of Sigma Phase
Many engineers are not able to ascertain that there is a difference between sigma phase and grain-boundary carbide formation, and that leads to wrong conclusions. Sigma phase is a precipitation product that can appear in both grains and grain boundaries. Examples of sigma-phase formation are shown in figures 2-4.[3]
Confusion arises when grain-boundary oxidation or carbide formation is observed in nickel and nominal nickel alloys, such as RA330®, that have a nominal nickel content of at least 35%. This level is sufficient to prevent sigma-phase formation. However, engineers mistake the oxidation for sigma phase.
Figure 5 shows RA330 that was exposed to 1900°F (1038°C) for 3,000 hours.[4] Even though 1900°F is well above the sigma formation range, some engineers concluded that the grain-boundary oxides were sigma phase. When there is any question, a metallurgist well versed in the metallography of these alloys should be consulted.
ADVERTISEMENT
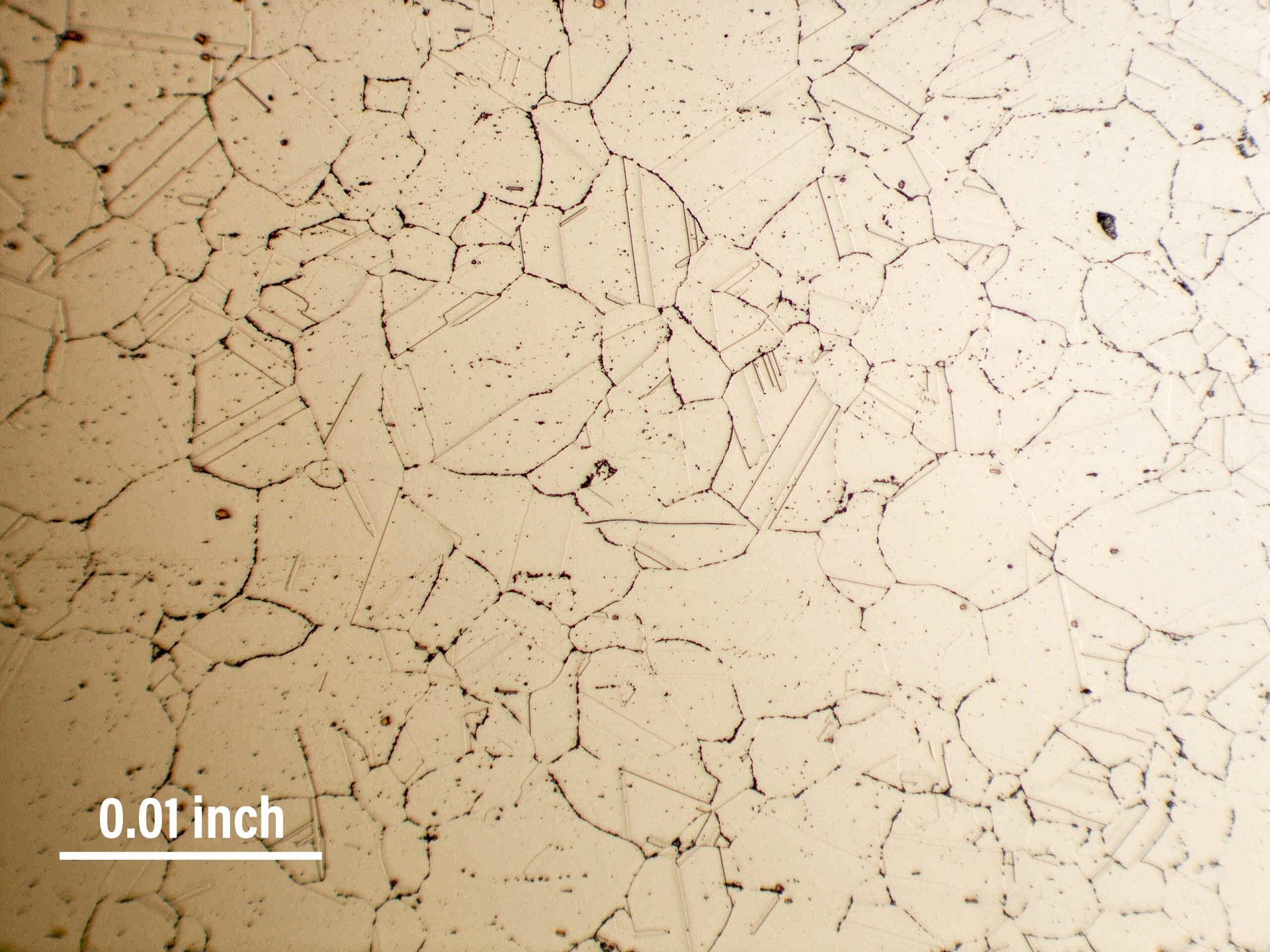
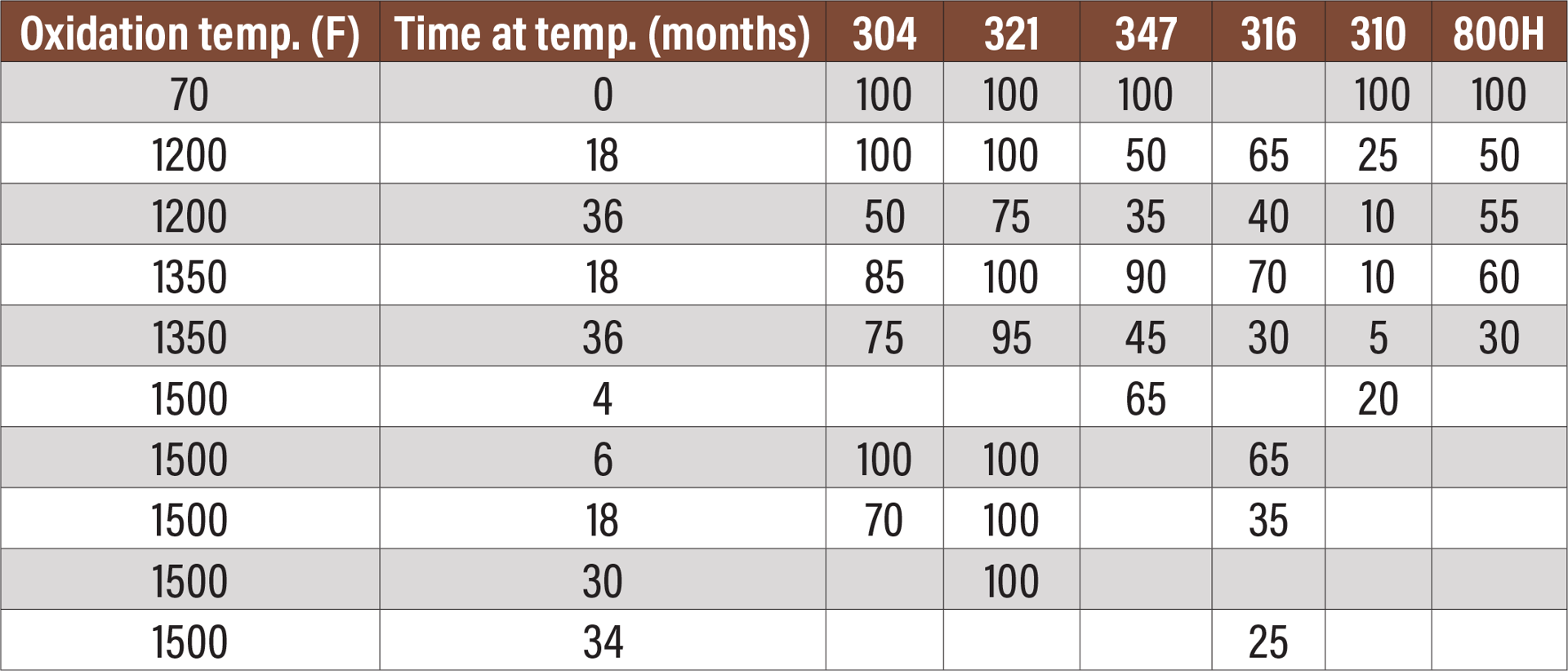
Fig. 5. RA330 at 1900°F for 3,000 hours. The oxides in the grain boundaries are confused by some with sigma phase. There is NO sigma phase here (100x).
Fig. 6. Room-temperature Charpy impact tests of various alloys oxidized at three temperatures for various times. All results are in foot-pounds.
Physical Properties of Material with and without Sigma Phase
Figure 6 is a table showing the results of impact testing of six different alloys oxidized at three different temperatures for varying times.[5] All alloys show some losses over time. Some alloys show significant losses and almost no ductility. Closer examination shows that each alloy has its own temperature where sigma phase forms the fastest. In fact, sigma-phase formation follows a C curve.
Figure 7 is a graph showing the aging curves for sigma-phase formation.[6] Anything to the right of the curve for a specific alloy will result in sigma-phase formation.
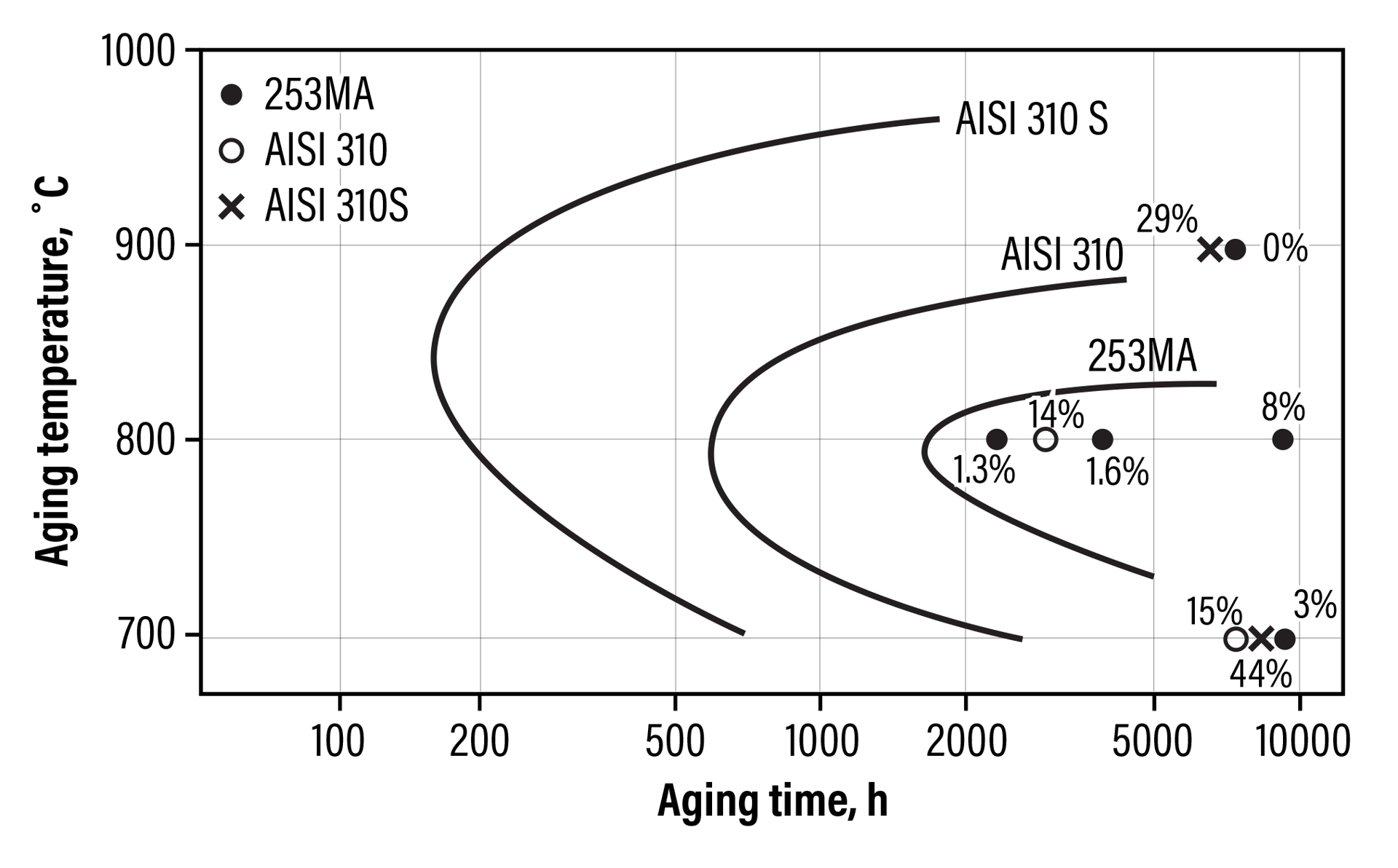
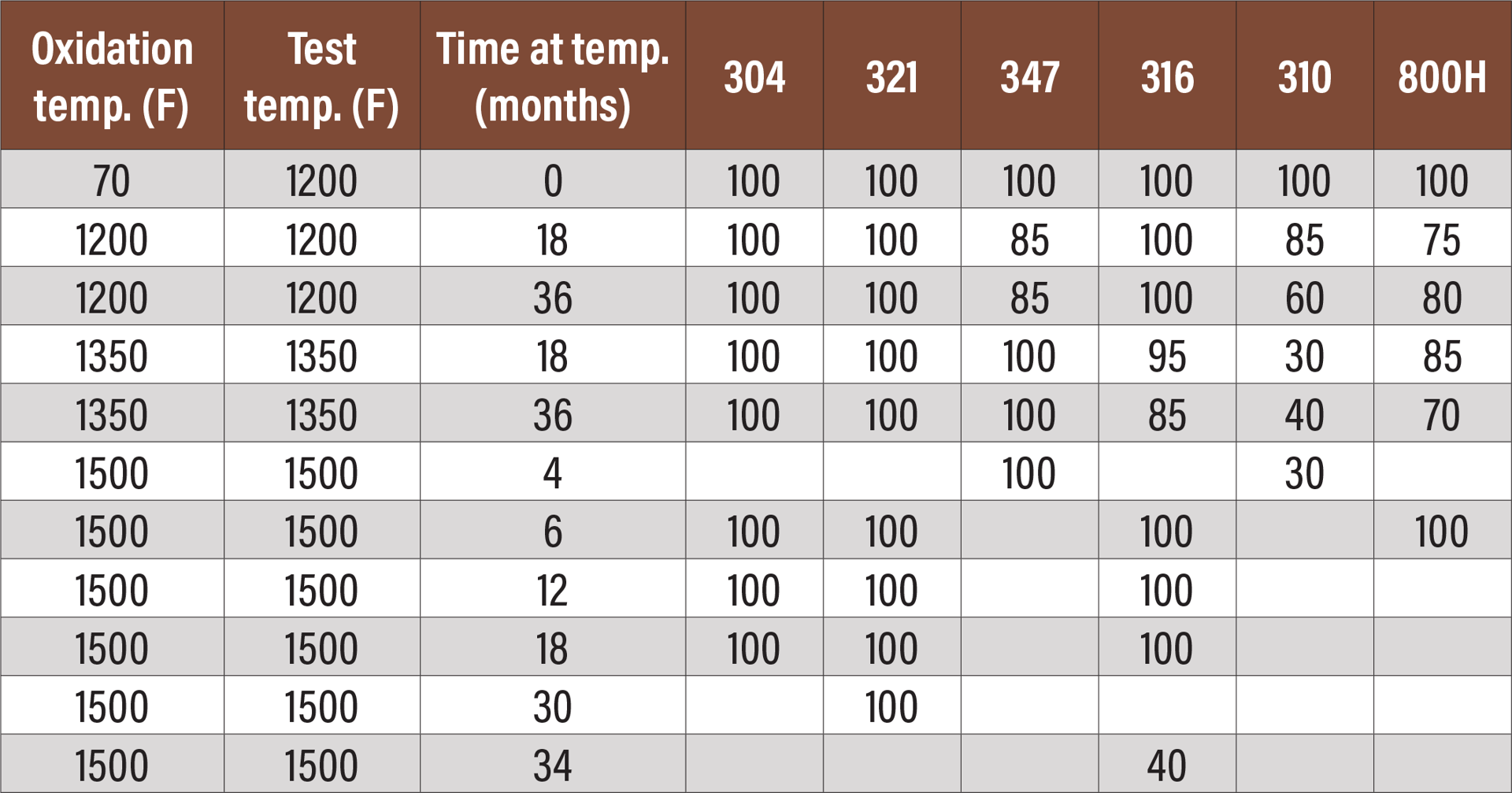
Fig. 7. Aging curves for the formation of sigma for various alloys.
Fig. 8. Elevated temperature Charpy impact tests of various alloys oxidized at three temperatures for various times. All results are in foot-pounds.
Figure 8 is a table showing the results of elevated temperature impact testing of the same six alloys.[5] This table shows either no loss of ductility when the test is performed at elevated temperature or significantly less loss of ductility. In most cases, there is still sufficient ductility that the material can be safely used at temperature.
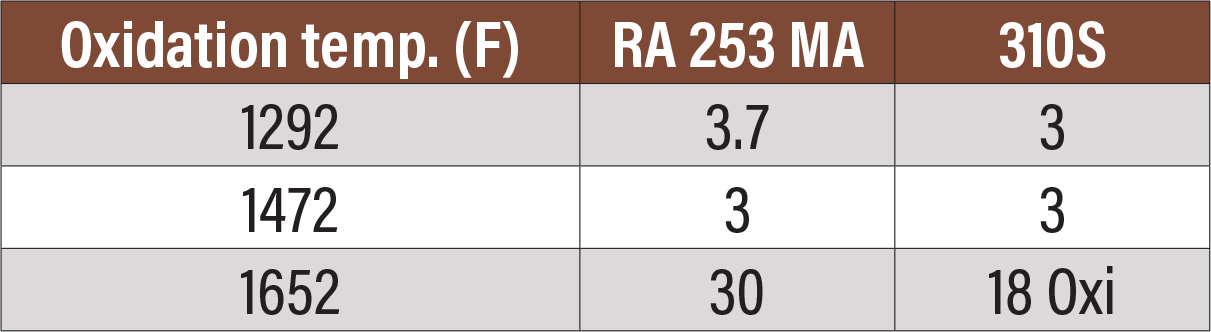
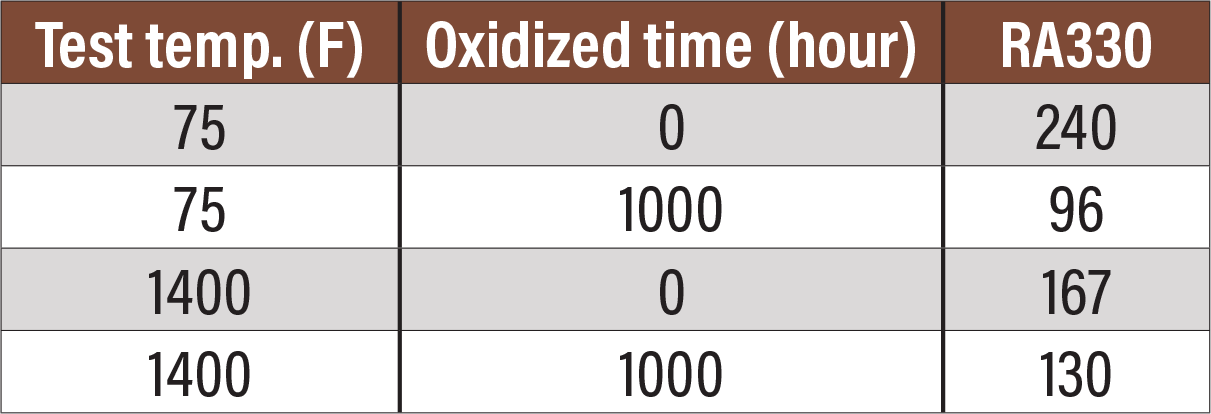
Fig. 9. Room-temperature Charpy impact testing of two alloys aged for 10,000 hours at three different temperatures.
Fig. 10. Charpy testing of RA330 as received and aged for 1,000 hours at 1400°F.
Figure 9 is a table showing the result of two alloys aged for 10,000 hours at various temperatures and Charpy tested at room temperature after aging.[7] There is significant loss of ductility.
Taken as a whole, these tables demonstrate that care should be taken to prevent any kind of impact when these alloys have formed sigma phase and then lowered to room temperature. At operating or heat-treating temperatures, these alloys (for the most part) retain all or sufficient ductility that they can be safely used.
Figure 10 is a table showing Charpy testing of RA330 after aging.[8] While there is still some loss of ductility, the energy absorbed in Charpy testing is sufficient that the material is quite ductile and safe for use at room temperature.
Conclusion
Sigma-phase precipitation occurs in stainless steels and alloys with less than 35% nominal nickel content. It does not occur in nickel alloys with 35% nickel or more. Sigma phase makes materials very brittle at room temperature. At elevated temperatures, within typical heat-treating ranges, most materials retain sufficient toughness to be used without concern. It should be noted, however, that there is loss in toughness even at elevated temperatures. More caution should be used in alloy selection in systems that vibrate because the constant vibration could be sufficient to cause premature failure if sigma phase has formed.
There have been instances where engineers mistake grain-boundary oxidation for sigma-phase formation in alloys that do not form sigma phase. Interpretations should be verified by metallurgists experienced in the metallography of stainless steels and nickel alloys to ensure sound conclusions.
253 MA® is a registered trademark of Outokumpu. RA 253 MA® and RA330® are registered trademarks of Rolled Alloys.
References
- Daniel H. Herring, “Sigma Phase Embrittlement,” Industrial Heating. BNP Media, Troy, MI. March 2012
- Binary Phase Diagrams. ASM International. Metals Park, OH. 1986
- Internal Reports. Rolled Alloys. Temperance, MI.
- Internal Reports. Rolled Alloys. Temperance, MI.
- George E. Lien. Behavior of Superheater Alloys in High Temperature, High Pressure Steam. The American Society of Mechanical Engineers, New York, NY. 1968
- Thomas Andersson and Thomas Odelstam. Sandvik 253MA (UNS S30815) – The Problem Solver for High Temperature Applications. R&D Centre AB Sandvik Steel Bulletin. Sandviken, Sweden. October 1984
- Rolled Alloys Bulletin 1353, RA 353 MA® Alloy
- Private Communications of January 10 and June 22, 1972, Crucible Inc., Materials Research Center
All images provided by author except where noted.
For more information: Contact Marc Glasser at Rolled Alloys, 125 West Sterns Road, Temperance, MI; tel: 800-521-0332, e-mail: metallurgical-help@rolledalloys.com; web: www.rolledalloys.com.


