meat science review
TECH

Fatty acid composition of meat animals as flavor precursors
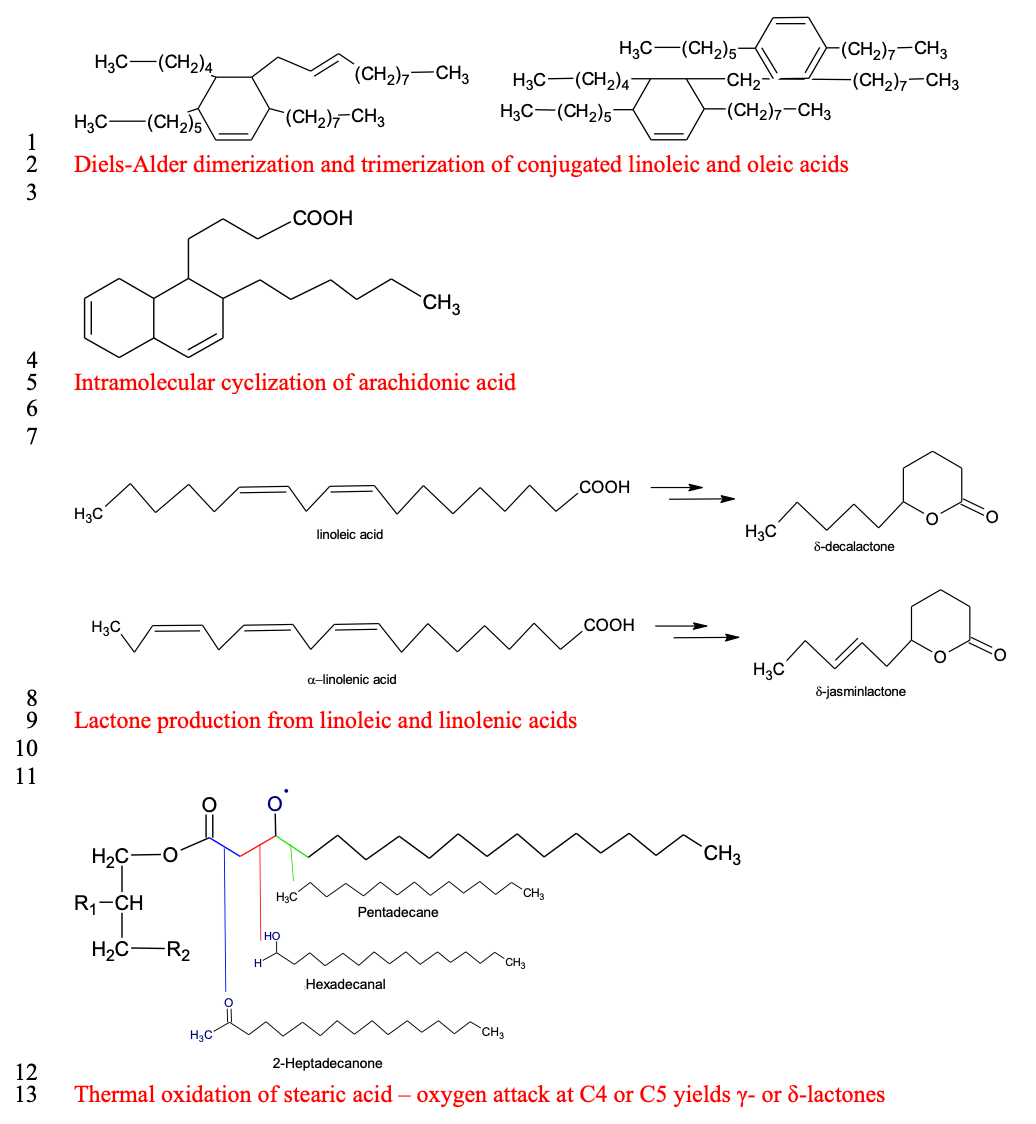
Thermal oxidation of fatty acids
Fat in meat animals consists mostly of triglycerides, acquired from dietary sources and fatty acid de novo synthesis (Bravo-Lamas et al., 2018). Lipid-derived flavor compounds include aldehydes (alkanals), ketones, carboxylic acids (alkanoics), alcohols (alkanols), lactones, and alkylfurans (Mottram, 1998). These compounds result from the oxidation of fatty acids in various chain reactions of free radicals. These reactions, if occurring during storage, lead to an undesirable aromatic profile and the end of shelf-life (Amaral et al., 2018). However, during cooking, similar reactions under thermal oxidation produce desirable and often characteristic cooked flavor profiles (Nawar, 1984; Song et al., 2011). During thermal oxidation, unsaturated fatty acids are oxidized more quickly (Song et al., 2011) and polymerized to produce more oxygenated dimers and polymers (Nawar, 1984; Mottram, 1998). In addition, short-chain aldehydes, ketones, and alcohols are further oxidized to organic acids and esters (Song et al., 2011), and lipid peroxides are polymerized to produce cyclic carboxylic acids and their lactones (cyclic carboxylic esters).
Saturated fatty acids (SFA) are more stable than unsaturated ones; however, during cooking at 150°C or above — such as on the surface of grilled steaks — SFA are oxidized in a more complex pattern, yielding long-chain alkanes, alkanals, ketones, and lactones (Nawar, 1984). This shift in volatile composition with less short-chain and unsaturated aldehydes and alcohols yields more desirable aromas, less volatility, and higher thresholds. Although thermal oxidation of lipids occurs at as low as 60°C, the desirable composition of lipid-derived volatiles is produced at temperatures from 100°C to 300°C (Wasserman, 1972) due to the rapid oxidation of unsaturated fatty acids and other oxidation products. Lipid oxidation products also interact with Maillard reaction products, which are formed when proteins begin to unfold at an internal temperature of 35°C and coagulate from 55°C to 80°C (Wasserman, 1972). When the meat surface is roasted at 190°C and fatty acids are thermally oxidized and polymerized, milder Maillard reactions occur at an internal temperature of 60°C to 80°C (Wasserman, 1972), in which lipid-derived aldehydes are active participants, yielding some of the most characteristic volatiles of cooked meat aroma.
By Thu T. N. Dinh1, K. Virellia To2, and M. Wes Schilling2
1Department of Animal and Dairy Science, Mississippi State University
2Department of Food Science, Nutrition, and Health Promotion, Mississippi State University
Figure 1: Thermal oxidation of fatty acids
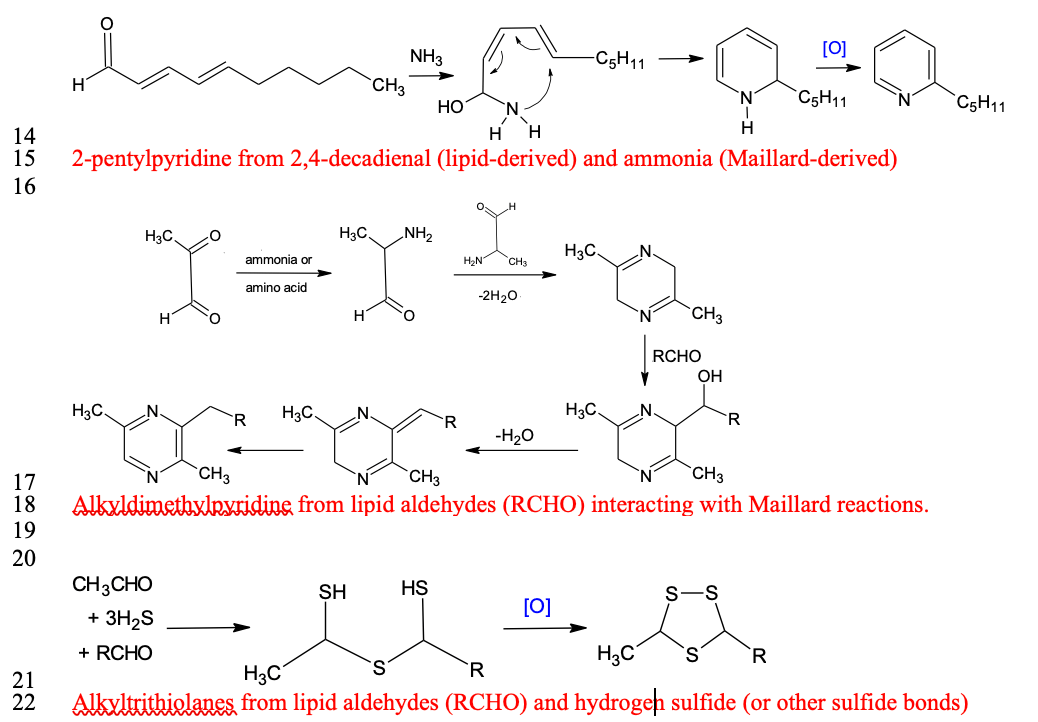
Interactions between lipid- and water-soluble flavor precursors
It has traditionally been accepted that lipids are the source of characteristic flavors in meat (Hornstein and Crowe, 1960), through the formation of saturated and unsaturated aldehydes with 6 to 10 carbons. However, lipid volatile compounds have greater thresholds than volatiles derived from water-soluble compounds such as those participating in Maillard reactions (Mottram, 1998). Moreover, the interactions between lipid-derived compounds and Maillard compounds are more important for the development of meat flavor than originally thought. Legako et al. (2016) reported that USDA Standard steaks produced more n-aldehydes than USDA Prime and Low-Choice steaks.
Although the percentage of polyunsaturated fatty acids (PUFA) in leaner meat is greater than that in fattier meat because of less total lipid content, Legako et al. (2015) reported that the PUFA content in USDA Standard steaks was still less than that in USDA Prime and Low-Choice steaks. Therefore, the development of lipid flavor compounds must also be influenced by the lean portion of meat, especially the water-soluble components. Lipid oxidation products that participate in Maillard reactions are most likely aldehydes that compete with carbonyls from reducing sugars for amino compounds (Zamora and Hidalgo, 2011). This competition produces characteristic and desirable flavor compounds in cooked meat (Zamora and Hidalgo, 2011; Kosowska et al., 2017).
Gardner and Legako (2018) found that dimethyl- and trimethyl pyrazine concentrations in the headspace of ground beef were greater at higher temperatures. Their data indicated that such an increase was more prevalent in USDA Prime beef than in USDA Choice and Standard. USDA Prime beef has more fat, and these pyrazines are products of lipid–Maillard interactions. These findings imply that fatty acid oxidation products are not simply flavor compounds; they are also precursors for complex interactions with Maillard products to form a more characteristic and desirable cooked meat flavor. Such interactions do not occur in autoxidation during the storage of meats.
Off-flavor compounds from lipid oxidation
During refrigerated storage at 1°C to 7°C, lipid oxidation occurs slowly with minimal interactions with other compounds such as Maillard products and yields various off-odor compounds that are detrimental to meat quality. The heat used in cooking accelerates lipid oxidation but also produces a desirable profile of lipid carbonyls and carboxylic acids for cooked meat flavor and facilitates the hydrolysis of fatty acids from triglycerides or phospholipids. This hydrolysis allows fatty acids to be more reactive and produce carbonyls more quickly (Wasserman, 1972). At lower storage temperatures, autoxidation of meat produces short-chain aldehydes and alcohols that have offensive odors (Ismail et al., 2008). Although aldehydes from the Maillard reactions such as 2,3-butanedione (Hunt et al., 2016) are desirable, major aldehydes from the autoxidation of meat lipids such as hexanal are undesirable.
Another phenomenon called “warmed-over,” coined first by Younathan and Watts (1959), describes the rancidity onset of cooked meat after a refrigeration period. The warmed-over flavor is caused by the continuous oxidation of lipids after cooking. Angelo et al. (1987) reported that warmed-over flavor was characterized by hexanal and 2,3‐octanedione, both of which are products of lipid oxidation. Studies in beef and pork have consistently identified these two compounds as warmed-over flavor markers. However, other lipid thermal oxidation products such as cyclic compounds are also oxidized and decomposed to offensive aromas during cooked meat storage.
Conclusions
Volatile compounds from autoxidation of unsaturated fatty acids causes off-odors, whereas the lipid-derived volatile profile is more desirable under thermal oxidation and in the reactions with flavor compounds such as Maillard reaction products. Recent research has suggested that the development of lipid flavor compounds is influenced by the lean portion of meat. Therefore, the interactions between lipid-derived compounds, water-soluble compounds, and Maillard compounds are likely more important than originally thought and warrant further research.
Figure 2: Interactions between lipid-derived and water-soluble flavor compounds
Literature Cited
Amaral, A. B., M. V. D. Silva, and S. C. D. S. Lannes. 2018. Lipid oxidation in meat: Mechanisms and protective factors–a review. Food Sci. Tech.-Brazil. 38:1–15. https://doi.org/10.1590/fst.32518.
Angelo, A. S., J. R. Vercellotti, M. G. Legendre, C. H. VinnelT, J. W. Kuan, C. James Jr., and H. P. Dupuy. 1987. Chemical and instrumental analyses of warmed‐over flavor in beef. J. Food Sci. 52:1163–1168. https://doi.org/10.1111/j.1365-2621.1987.tb14034.x.
Bravo-Lamas, L., L. J. Barron, L. Farmer, and N. Aldai. 2018. Fatty acid composition of intramuscular fat and odour-active compounds of lamb commercialized in northern
Gardner, K., and J. F. Legako. 2018. Volatile flavor compounds vary by beef product type and degree of doneness. J. Anim. Sci. 96:4238–4250. https://doi.org/10.1093/jas/sky287.
Hornstein, I., and P. F. Crowe. 1960. Meat flavor chemistry, flavor studies on beef and pork. J. Agr. Food Chem. 8:494–498. https://doi.org/10.1021/jf60112a022.
Hunt, M. R., J. F. Legako, T. T. N. Dinh, A. J. Garmyn, T. G. O’Quinn, C. H. Corbin, R. J. Rathmann, J. C. Brooks, and M. F. Miller. 2016. Assessment of volatile compounds, neutral and polar lipid fatty acids of four beef muscles from USDA Choice and Select graded carcasses and their relationships with consumer palatability scores and intramuscular fat content. Meat Sci. 116:91–101. https://doi.org/10.1016/j.meatsci.2016.02.010.
Ismail, H. A., E. J. Lee, K. Y. Ko, and D. U. Ahn. 2008. Effects of aging time and natural antioxidants on the color, lipid oxidation and volatiles of irradiated ground beef. Meat Sci. 80:582–591. https://doi.org/10.1016/j.meatsci.2008.02.007.
Kosowska, M., M. A. Majcher, and T. Fortuna. 2017. Volatile compounds in meat and meat products. Food Sci. Tech.-Brazil. 37:1–7. https://doi.org/10.1590/1678-457X.08416.
Legako, J. F., T. T. N. Dinh, M. F. Miller, K. Adhikari, and J. C. Brooks. 2016. Consumer palatability scores, sensory descriptive attributes, and volatile compounds of grilled beef steaks from three USDA Quality Grades. Meat Sci. 112:77–85. https://doi.org/10.1016/j.meatsci.2015.10.018.
Legako, J. F., T. T. N. Dinh, M. F. Miller, and J. C. Brooks. 2015. Effects of USDA beef quality grade and cooking on fatty acid composition of neutral and polar lipid fractions. Meat Sci. 100:246–255. https://doi.org/10.1016/j.meatsci.2014.10.013.
Mottram, D. S. 1998. Flavour formation in meat and meat products: A review. Food
Nawar, W. W. 1984. Chemical changes in lipids produced by thermal processing. J. Chem. Educ. 61:299. https://doi.org/10.1021/ed061p299.
Song, S., X. Zhang, K. Hayat, P. Liu, C. Jia, S. Xia, Z. Xiao, H. Tian, and Y. Niu. 2011. Formation of the beef flavour precursors and their correlation with chemical parameters during the controlled thermal oxidation of tallow. Food Chem. 124:203–209. https://doi.org/10.1016/j.foodchem.2010.06.010.
Wasserman, A. E. 1972. Thermally produced flavor components in the aroma of meat and poultry. J. Agr. Food Chem. 20:737–741. https://doi.org/10.1111/j.1365-2621.1970.tb12177.x.
Younathan, M. T., and B. M. Watts. 1959. Relationship of meat pigments to lipid oxidation. J. Food Sci. 6:728–734. https://doi.org/10.1111/j.1365-2621.1959.tb17326.x.
Zamora, R., and F. J. Hidalgo. 2011. The Maillard reaction and lipid oxidation. Lipid Technology. 23:59–62. https://doi.org/10.1002/lite.201100094.
